
Chemistry, 21.05.2020 18:00 NathanaelLopez
Carbon disulfide is prepared by heating sulfur and charcoal. The chemical equation is ; =9.40 at 900 K How many grams of CS₂(g) can be prepared by heating 13.8 mol S₂(g) with excess carbon in a 8.60 L reaction vessel held at 900 K until equilibrium is attained?
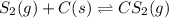

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
Carbon disulfide is prepared by heating sulfur and charcoal. The chemical equation is ; =9.40 at 900...
Questions

Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

History, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30




Social Studies, 18.03.2021 02:30


English, 18.03.2021 02:30



Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

Mathematics, 18.03.2021 02:30

Computers and Technology, 18.03.2021 02:30


Biology, 18.03.2021 02:30



