
Chemistry, 22.05.2020 01:00 ayoismeisjjjjuan
At high temperatures, calcium carbonate (CaCO3) decomposes to form calcium oxide (CaO) and carbon dioxide (CO2) gas. The average mass of 1 calcium oxide molecule is 56.079 amu, and the average mass of 1 carbon dioxide molecule is 44.009 amu.
CaCO3 CaO + CO2
Which is the total mass of the 1 calcium carbonate molecule?
A. 12.070 amu
B. 44.009 amu
C. 56.079 amu
D. 100.088 amu

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3


Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
At high temperatures, calcium carbonate (CaCO3) decomposes to form calcium oxide (CaO) and carbon di...
Questions





Mathematics, 06.01.2021 18:50





Mathematics, 06.01.2021 18:50


Business, 06.01.2021 18:50



Mathematics, 06.01.2021 18:50

Mathematics, 06.01.2021 18:50

Mathematics, 06.01.2021 18:50


Mathematics, 06.01.2021 18:50

Mathematics, 06.01.2021 18:50

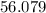 amu
amu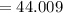 amu
amu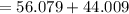
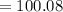 amu
amu

