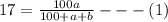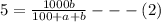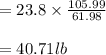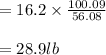Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 ). During heating, these two ingredients decompose to give off carbon dioxide (CO2 ), the resulting products being soda and lime. Compute the weight of soda ash and limestone that must be added to 125 lbm of quartz (SiO2 ) to yield a glass of composition 78 wt% SiO2 , 17 wt% Na2O, and 5 wt% CaO.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1


Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Soda and lime are added to a glass batch in the form of soda ash (Na2CO3 ) and lime-stone (CaCO3 )....
Questions

Biology, 09.12.2020 21:30




Mathematics, 09.12.2020 21:30

Mathematics, 09.12.2020 21:30

Mathematics, 09.12.2020 21:30

History, 09.12.2020 21:30

Mathematics, 09.12.2020 21:30

History, 09.12.2020 21:30


Social Studies, 09.12.2020 21:30

Arts, 09.12.2020 21:30




Chemistry, 09.12.2020 21:30


Mathematics, 09.12.2020 21:30