
Chemistry, 28.05.2020 20:04 roseemariehunter12
Acetic acid (HC2H3O2, Ka=1.8x10^-5) is a weak acid. Calculate the pH of an aqueous solution of .25M acetic acid.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
Acetic acid (HC2H3O2, Ka=1.8x10^-5) is a weak acid. Calculate the pH of an aqueous solution of .25M...
Questions

Mathematics, 26.12.2019 06:31


Social Studies, 26.12.2019 06:31



Mathematics, 26.12.2019 06:31

Mathematics, 26.12.2019 06:31

Mathematics, 26.12.2019 06:31

Mathematics, 26.12.2019 06:31

History, 26.12.2019 06:31

Mathematics, 26.12.2019 06:31

Physics, 26.12.2019 06:31






History, 26.12.2019 06:31

History, 26.12.2019 06:31

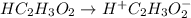
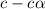
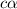
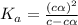
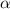 = ?
= ?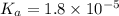
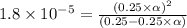
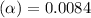
![[H^+]=c\times \alpha](/tpl/images/0669/0176/4fc41.png)
![[H^+]=0.25\times 0.0084=0.0021](/tpl/images/0669/0176/aff35.png)
![pH=-log[H^+]](/tpl/images/0669/0176/15713.png)
![pH=-log[0.0021]=2.7](/tpl/images/0669/0176/5e099.png)


