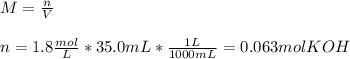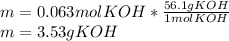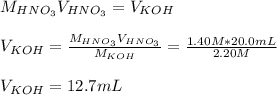Chemistry, 27.05.2020 23:01 sneakersolequeen
PLEASE SHOW WORK
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the concentration of the acetic acid were 2.40 M, what would be the concentration of H + at equilibrium?
2) You have a solution that is 1.75 M HCN. If the K a is 9.9 × 10 –8 , calculate the pH of the solution.
3) How many grams of KOH are needed to neutralize 35.0 mL of 1.8 M HCl?
4) How many mL of 2.20 M KOH are needed to neutralize 20.0 mL of 1.40 M HNO 3 ?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
PLEASE SHOW WORK
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the...
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the...
Questions

English, 21.10.2020 17:01


History, 21.10.2020 17:01


Mathematics, 21.10.2020 17:01














English, 21.10.2020 17:01

![[H^+]_{eq}=0.00029M](/tpl/images/0667/7635/6d441.png)
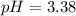
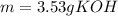
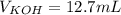
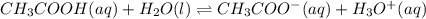
![Ka=\frac{[CH_3COO^-][H_3O^+]}{[CH_3COOH]}](/tpl/images/0667/7635/3cdd5.png)
 due to reaction's extent we have:
due to reaction's extent we have: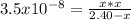
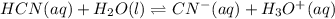
![K=\frac{[CN^-][H_3O^+]}{[HCN]}](/tpl/images/0667/7635/eab5d.png)
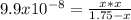
![[H^+]_{eq}=0.000416 M](/tpl/images/0667/7635/3b132.png)
![pH=-log([H^+]_{eq})=-log(0.000416)\\\\pH=3.38](/tpl/images/0667/7635/c9e0e.png)