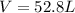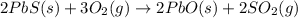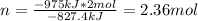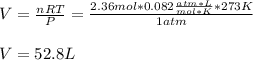Chemistry, 29.05.2020 05:59 Mistytrotter
2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g) H = -827.4 kJ What is the volume of sulfur dioxide produced at STP if 975 kJ of heat are liberated?

Answers: 3
Another question on Chemistry

Chemistry, 20.06.2019 18:02
Chemistry do these i am so stressed i have 14 essays due tomorrow
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g) H = -827.4 kJ What is the volume of sulfur dioxide produced at...
Questions

Mathematics, 17.11.2020 14:00

History, 17.11.2020 14:00

Mathematics, 17.11.2020 14:00

History, 17.11.2020 14:00

History, 17.11.2020 14:00



Mathematics, 17.11.2020 14:00


Mathematics, 17.11.2020 14:00

History, 17.11.2020 14:00

English, 17.11.2020 14:00

Computers and Technology, 17.11.2020 14:00

English, 17.11.2020 14:00




Advanced Placement (AP), 17.11.2020 14:00

Chemistry, 17.11.2020 14:00

Mathematics, 17.11.2020 14:00