
Chemistry, 01.06.2020 15:58 babygirl091502
What is the molarity of a solution formed by dissolving 100 g of Ca(No3)2 and 450 mL of solution
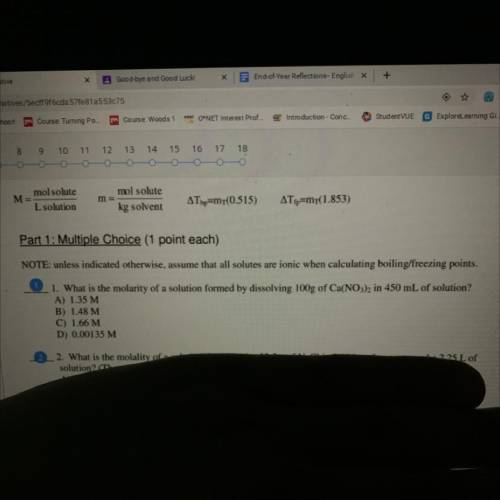

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
What is the molarity of a solution formed by dissolving 100 g of Ca(No3)2 and 450 mL of solution
Questions

Mathematics, 14.10.2019 20:30

History, 14.10.2019 20:30



Mathematics, 14.10.2019 20:30






Spanish, 14.10.2019 20:30

Health, 14.10.2019 20:30




Geography, 14.10.2019 20:30

Chemistry, 14.10.2019 20:30



Social Studies, 14.10.2019 20:30



