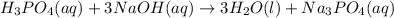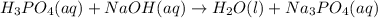Chemistry, 02.06.2020 23:00 angelinamadriga
In a neutralization reaction with a strong acid or base, all of the hydrogens from the acid and the hydroxides from the base react to form water.
Balance the following neutralization reaction:
H3PO4 (aq) + NaOH (aq) â H2O (l) + Na3PO4 (aq)
Enter the appropriate coefficient to the left of the respective term. In the event that your coefficient would be "1" be sure to write "1" as opposed to leaving it blank.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1


Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
In a neutralization reaction with a strong acid or base, all of the hydrogens from the acid and the...
Questions

Social Studies, 18.07.2020 21:01















Biology, 18.07.2020 21:01


Mathematics, 18.07.2020 21:01