
Chemistry, 06.06.2020 01:59 jpimentel2021
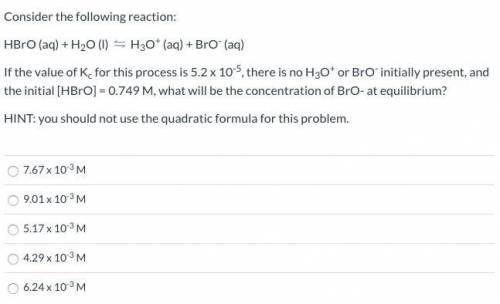
HBrO (aq) + H2O (l) ⇋ H3O+ (aq) + BrO- (aq)
If the value of Kc for this process is 5.2 x 10-5, there is no H3O+ or BrO- initially present, and the initial [HBrO] = 0.749 M, what will be the concentration of BrO- at equilibrium?
HINT: you should not use the quadratic formula for this problem.
7.67 x 10-3 M
9.01 x 10-3 M
5.17 x 10-3 M
4.29 x 10-3 M
6.24 x 10-3 M

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2


Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
HBrO (aq) + H2O (l) ⇋ H3O+ (aq) + BrO- (aq)
If the value of Kc for this process is 5.2 x 10-5, ther...
Questions





Mathematics, 05.12.2020 07:20

Mathematics, 05.12.2020 07:20


Social Studies, 05.12.2020 07:20


Mathematics, 05.12.2020 07:20




Mathematics, 05.12.2020 07:20

Mathematics, 05.12.2020 07:20

Mathematics, 05.12.2020 07:20

Mathematics, 05.12.2020 07:20



Social Studies, 05.12.2020 07:20



