
Chemistry, 12.06.2020 02:57 eddsworldfrantic
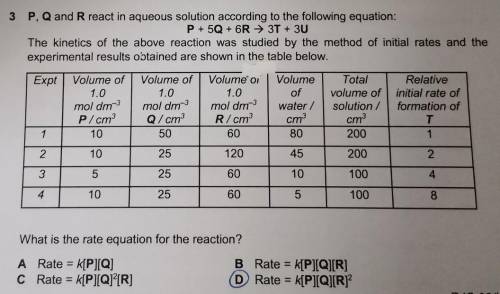
P, Q and R react in aqueous solution according to the following equation:
P + 50 + 6R → 3T + 3U
The kinetics of the above reaction was studied by the method of initial rates and the
experimental results obtained are shown in the table below
MCQ question (reaction kinetics)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 07:30
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
You know the right answer?
P, Q and R react in aqueous solution according to the following equation:
P + 50 + 6R → 3T + 3U
Questions



Mathematics, 04.02.2021 01:50

Mathematics, 04.02.2021 01:50



Arts, 04.02.2021 01:50


Mathematics, 04.02.2021 01:50




English, 04.02.2021 01:50



English, 04.02.2021 01:50




Biology, 04.02.2021 01:50



