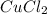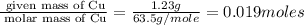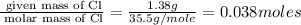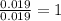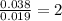Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Which organic molecule is found in the chromatin of cells?
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Copper metal of 1.23 g sample is reacted completely with chlorine gas to produce
2.61 g of copper c...
Questions

Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01







Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

English, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01


Social Studies, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

History, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01