
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+Pi⟶glucose-6-phosphate+H2 OATP+H2O⟶ADP+PiΔG=+13.8 kJ/molΔG=−30.5 kJ/mol reaction 1:glucose+Pi⟶glucose-6-phosphate+H2 OΔG=+13.8 kJ/molreaction 2:ATP+H2O⟶ADP+PiΔG=−30.5 kJ/mol Answer the four questions about the first step of glycolysis. Is reaction 2 spontaneous or nonspontaneous?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1


Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+...
Questions

History, 21.02.2020 20:28

Mathematics, 21.02.2020 20:28

Computers and Technology, 21.02.2020 20:28


English, 21.02.2020 20:29



Mathematics, 21.02.2020 20:30

Mathematics, 21.02.2020 20:30

Mathematics, 21.02.2020 20:30



Mathematics, 21.02.2020 20:31

Computers and Technology, 21.02.2020 20:31




Health, 21.02.2020 20:32


Mathematics, 21.02.2020 20:32

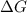 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol
glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol

