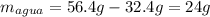Chemistry, 18.06.2020 19:57 amiahmiller79
La masa de un recipiente con tapa es 32,4g y cuando se le coloca agua en su interior su masa es de 56,4g. Se introduce el recipiente en el freezer hasta que se forme hielo. a) ¿Cuál es la masa de agua contenida en el recipiente? b) ¿Cuál será la masa del recipiente con el hielo? Justifica. c) ¿Se produjo un proceso físico o químico? ¿Por qué? d) Investiga ¿qué es la fusión?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
La masa de un recipiente con tapa es 32,4g y cuando se le coloca agua en su interior su masa es de 5...
Questions

History, 27.01.2021 15:00

Business, 27.01.2021 15:00


Mathematics, 27.01.2021 15:00

History, 27.01.2021 15:00



Mathematics, 27.01.2021 15:00






English, 27.01.2021 15:00

Mathematics, 27.01.2021 15:00


Social Studies, 27.01.2021 15:10

Arts, 27.01.2021 15:10

Mathematics, 27.01.2021 15:10

Mathematics, 27.01.2021 15:10