Rxn
Use the ΔΗ° and ΔΗ°, information provided to calculate AH°F for SO3(g):
AH f (kJ/mol) 2 S...

Chemistry, 23.06.2020 10:57 lolorichards123
Rxn
Use the ΔΗ° and ΔΗ°, information provided to calculate AH°F for SO3(g):
AH f (kJ/mol) 2 SO2(g) + O2(g) → 2 SO3(9) AH°rxn = -198 kJ
SO2(g) -297

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1


Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Questions

Mathematics, 19.05.2021 21:50



Social Studies, 19.05.2021 21:50



Mathematics, 19.05.2021 21:50




History, 19.05.2021 21:50

Mathematics, 19.05.2021 21:50

History, 19.05.2021 21:50

Biology, 19.05.2021 21:50

Mathematics, 19.05.2021 21:50

Mathematics, 19.05.2021 21:50


Mathematics, 19.05.2021 21:50

Chemistry, 19.05.2021 21:50

Physics, 19.05.2021 21:50

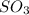 is -396 kJ/mol
is -396 kJ/mol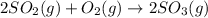
![\Delta H^o_{rxn}=[(2\times \Delta H^o_f_{(SO_3(g))})]-[(2\times \Delta H^o_f_{(SO_2(g))})+(1\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0692/0702/55e29.png)
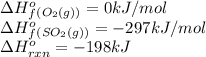
![-198=[(2\times \Delta H^o_f_{(SO_3(g))})]-[(2\times \Delta -297)+(1\times (0))]\\\\\Delta H^o_f_{(SO_3(g))}=-396kJ/mol](/tpl/images/0692/0702/d13c7.png)



