
Chemistry, 08.07.2020 02:01 kingsqueen883
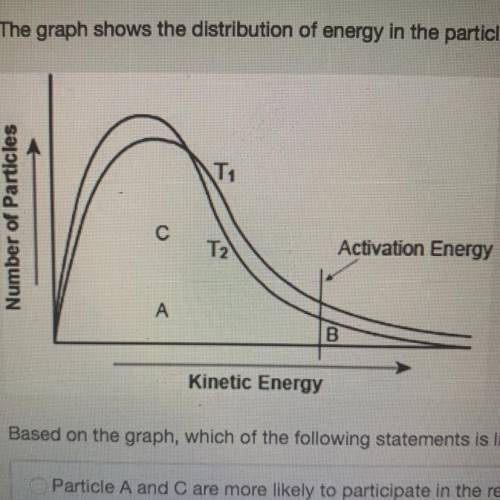
The graph shows the distribution of energy in the particles of two gas samples at different temperatures, T, and T2. A, B, and C represent individual particles.
Number of Particles
Activation Energy
Kinetic Energy
Based on the graph, which of the following statements is likely to be true?
Particle A and C are more likely to participate in the reaction than particle B.
Most of the particles of the two gases have very high speeds.
A fewer number of particles of gas at T, are likely to participate in the reaction than the gas at T2
The average speed of gas particles at Te is lower than the average speed of gas particles at Ty

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
The graph shows the distribution of energy in the particles of two gas samples at different temperat...
Questions


Chemistry, 30.04.2021 21:20


Mathematics, 30.04.2021 21:20


History, 30.04.2021 21:20


Mathematics, 30.04.2021 21:20





Social Studies, 30.04.2021 21:20


Mathematics, 30.04.2021 21:20

Mathematics, 30.04.2021 21:20

Mathematics, 30.04.2021 21:20


Law, 30.04.2021 21:20

Chemistry, 30.04.2021 21:20



