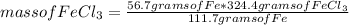Chemistry, 17.07.2020 01:01 chantianabess36
For the reaction shown, calculate the number of grams of product from 56.7 grams of iron and sufficient chlorine. Enter your answer with the correct significant figures. Do not include units with your answer. 2Fe (s) + 3Cl2 (g) = 2FeCl3 (s)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
For the reaction shown, calculate the number of grams of product from 56.7 grams of iron and suffici...
Questions




Mathematics, 06.07.2019 00:30

Mathematics, 06.07.2019 00:30


Mathematics, 06.07.2019 00:30








English, 06.07.2019 00:30


History, 06.07.2019 00:30


Mathematics, 06.07.2019 00:30