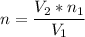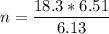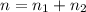Chemistry, 16.07.2020 17:01 silasjob09
A flexible container at an initial volume of 6.13 L contains 6.51 mol of gas. More gas is then added to the container until it reaches a final volume of 18.3 L. Assuming the pressure and temperature of the gas remain constant, calculate the number of moles of gas added to the container.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1



Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
A flexible container at an initial volume of 6.13 L contains 6.51 mol of gas. More gas is then added...
Questions

Mathematics, 11.10.2020 07:01

Social Studies, 11.10.2020 07:01

Mathematics, 11.10.2020 07:01


Mathematics, 11.10.2020 07:01

Mathematics, 11.10.2020 07:01


Social Studies, 11.10.2020 07:01

World Languages, 11.10.2020 07:01

Chemistry, 11.10.2020 07:01

Social Studies, 11.10.2020 07:01

Arts, 11.10.2020 07:01


Social Studies, 11.10.2020 07:01


Physics, 11.10.2020 07:01


French, 11.10.2020 07:01


Business, 11.10.2020 07:01