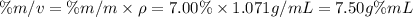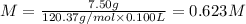Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
What is the molarity of a solution that is 7.00% by mass magnesium sulfate and has a density of 1.07...
Questions


English, 22.06.2019 09:00

Social Studies, 22.06.2019 09:00




English, 22.06.2019 09:00



English, 22.06.2019 09:00



Mathematics, 22.06.2019 09:00

Chemistry, 22.06.2019 09:00

Chemistry, 22.06.2019 09:00



Biology, 22.06.2019 09:00

Mathematics, 22.06.2019 09:00