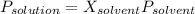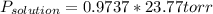Chemistry, 27.07.2020 01:01 wolfking800
The vapor pressure of pure water at 250C is 23.77 torr. What is the vapor pressure of water above a solution that is 1.500 m glucose, C6H12O6?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
The vapor pressure of pure water at 250C is 23.77 torr. What is the vapor pressure of water above a...
Questions


Mathematics, 19.11.2019 08:31

Health, 19.11.2019 08:31

History, 19.11.2019 08:31

History, 19.11.2019 08:31



English, 19.11.2019 08:31



Mathematics, 19.11.2019 08:31

Mathematics, 19.11.2019 08:31

Mathematics, 19.11.2019 08:31

Mathematics, 19.11.2019 08:31

Mathematics, 19.11.2019 08:31


Mathematics, 19.11.2019 08:31

Mathematics, 19.11.2019 08:31