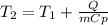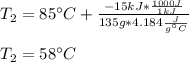Chemistry, 12.08.2020 07:01 FlowerChild1229
A 135 g sample of H20 at 85°C is cooled. The water loses a total of 15 kJ of energy in the cooling
process. What is the final temperature of the water? The specific heat of water is 4.184 J/g.°C.
A. 112°C
B. 58°C
C. 70°C
D. 84°C
E. 27°C

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
A 135 g sample of H20 at 85°C is cooled. The water loses a total of 15 kJ of energy in the cooling...
Questions


Computers and Technology, 14.01.2021 16:40



Chemistry, 14.01.2021 16:40




Social Studies, 14.01.2021 16:40



Mathematics, 14.01.2021 16:40




Chemistry, 14.01.2021 16:40

Social Studies, 14.01.2021 16:40



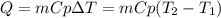
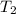 we obtain:
we obtain: