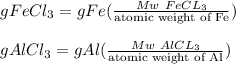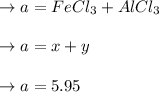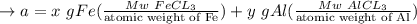Chemistry, 15.08.2020 23:01 dameiranderson
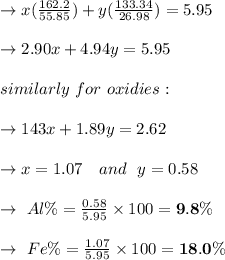
A mixture containing only FeCl3 and AlClz weighs 5.95 g . The chlorides are converted to the hydrous oxides and ignited to Fe2O3 and Al2O3 . The oxide mixture weighs 2.62 g . Calculate the percent Fe and Al in the original mixture .

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
A mixture containing only FeCl3 and AlClz weighs 5.95 g . The chlorides are converted to the hydrous...
Questions

Biology, 05.02.2021 22:00




Mathematics, 05.02.2021 22:00



Computers and Technology, 05.02.2021 22:00

English, 05.02.2021 22:00

Physics, 05.02.2021 22:00

Arts, 05.02.2021 22:00




Mathematics, 05.02.2021 22:00

Biology, 05.02.2021 22:00

History, 05.02.2021 22:00


Mathematics, 05.02.2021 22:00

Mathematics, 05.02.2021 22:00

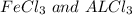 = 5.95g
= 5.95g