
Chemistry, 18.08.2020 17:01 cluchmasters5634
Consider the following reaction at equilibrium. What effect will increasing the pressure of the reaction mixture have on the system?
CuS(s) + O2(g) ? Cu(s) + SO2(g)
Consider the following reaction at equilibrium. What effect will increasing the pressure of the reaction mixture have on the system?
CuS(s) + O2(g) ? Cu(s) + SO2(g)
a) The equilibrium constant will increase.
b) The reaction will shift to the left in the direction of reactants.
c) The reaction will shift to the right in the direction of products.
d) No effect will be observed.
e) The equilibrium constant will decrease.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

You know the right answer?
Consider the following reaction at equilibrium. What effect will increasing the pressure of the reac...
Questions







Mathematics, 07.05.2020 00:12


Chemistry, 07.05.2020 00:12

Mathematics, 07.05.2020 00:12




Mathematics, 07.05.2020 00:12

History, 07.05.2020 00:12





![Q = \displaystyle \frac{[\mathrm{SO_2\, (g)}]}{[\mathrm{O_2\, (g)}]}](/tpl/images/0723/8610/a7d3d.png) .
.![[\mathrm{SO_2\, (g)}]](/tpl/images/0723/8610/2691f.png) and
and ![[\mathrm{O_2\, (g)}]](/tpl/images/0723/8610/46794.png) will increase if the pressure is increased through compression. However, because
will increase if the pressure is increased through compression. However, because 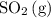 and
and 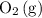 have the same coefficients in the equation, their concentrations are raised to the same power in the equilibrium quotient
have the same coefficients in the equation, their concentrations are raised to the same power in the equilibrium quotient 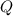 .
. 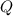 . If the system was already at equilibrium, it will continue to be at an equilibrium even after the change to its pressure. Therefore, no overall effect on the equilibrium position should be visible.
. If the system was already at equilibrium, it will continue to be at an equilibrium even after the change to its pressure. Therefore, no overall effect on the equilibrium position should be visible.

