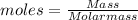Chemistry, 21.08.2020 14:01 pennyluvsu13
The molar mass of silver (Ag) is 107.87 g/mol.
Calculate the mass in grams of a sample of Ag containing 1.97 x 1022 atoms.
Write your answer using three significant figures.
g Ag

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
The molar mass of silver (Ag) is 107.87 g/mol.
Calculate the mass in grams of a sample of Ag contai...
Questions




Mathematics, 05.03.2021 07:20

Mathematics, 05.03.2021 07:20

Biology, 05.03.2021 07:30


Mathematics, 05.03.2021 07:30


Mathematics, 05.03.2021 07:30

Mathematics, 05.03.2021 07:30


History, 05.03.2021 07:30


Mathematics, 05.03.2021 07:30

History, 05.03.2021 07:30


Mathematics, 05.03.2021 07:30