
Chemistry, 25.08.2020 01:01 HalpMahOnMahH0meW0rk
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate.
a)Calculate the volume of gas measured at r. t.p, produced in this reaction
b)calculatr the maximum mass of copper(ii)nitrate, produced in this reaction
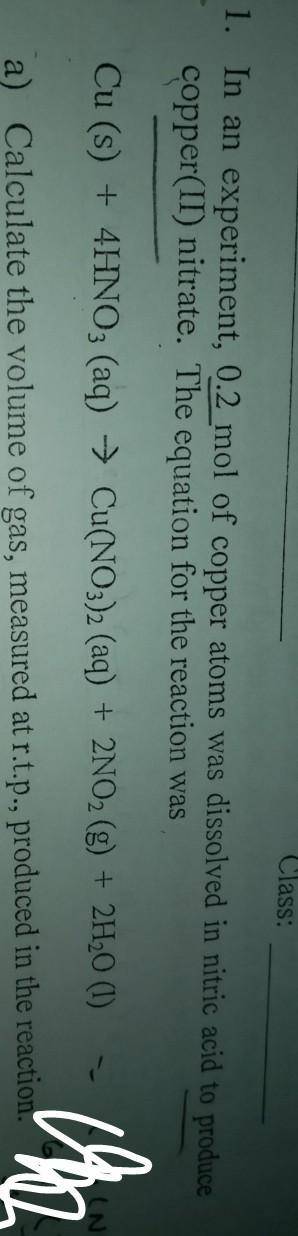

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1


Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate....
Questions






Computers and Technology, 09.12.2019 18:31










Social Studies, 09.12.2019 18:31






