

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 15:20
Plzzz ? which stores information in discrete steps? a magnet and coil of wire compact discs plastic records amplified speakers
Answers: 2

Chemistry, 23.06.2019 17:30
What amount in moles does 242 l of carbon dioxide occupy at 1.32 atm and 20 degrees c?
Answers: 2

Chemistry, 23.06.2019 22:50
When 10.g of ch3cooh is combusted in a sealed calorimeter, it releases enough energy to heat 2000. g of water from 23.5 °c to 34.3 °c. a. calculate the energy released per 10 g of ch3cooh. b. calculate the energy released per mole of ch3cooh.
Answers: 2

Chemistry, 24.06.2019 02:20
Sharon reads two different articles about avocados. the first article, in a weight loss magazine, claims that avocados are unhealthy because they are high in fat. the second article, in an annual publication from a doctors’ group, claims that the fat in avocados is a type that is good for the heart. to assess these claims, which question(s) might sharon ask? check all that apply. is the source of this claim reputable? where are avocados sold? is the source an authority on the subject? which source do avocados come from? how much do avocados cost?
Answers: 1
You know the right answer?
For the reaction 3C2H2(g)---> C6H6(l) at 25 C the standard enthalpy change is -631 kj and the sta...
Questions


Mathematics, 20.04.2020 20:49


Biology, 20.04.2020 20:49

Mathematics, 20.04.2020 20:49

Spanish, 20.04.2020 20:49


Physics, 20.04.2020 20:49


Geography, 20.04.2020 20:49

Mathematics, 20.04.2020 20:49









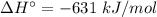 .
.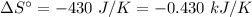 .
.![\Delta G^o=\Delta H^o-T\Delta S^o\\\\\Delta G^o=-631-[298\times (-0.430)]\ kJ\\ \\\Delta G^o=-631-(-128.14)\ kJ\\\\\Delta G^o=-502.86\ kJ](/tpl/images/0770/6490/cfc98.png)


