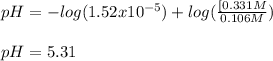A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate the pH of the solution following the addition of 0.061 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The Ka of butanoic acid is 1.52×10−5 .

Answers: 3
Another question on Chemistry

Chemistry, 20.06.2019 18:04
Asample of rose gold is: 12.0 g gold, 5.0g silver, and 7.0 g copper. what is the percent copper in the sample?
Answers: 2



Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
A 1.41 L buffer solution consists of 0.149 M butanoic acid and 0.288 M sodium butanoate. Calculate t...
Questions


English, 16.06.2020 21:57


Health, 16.06.2020 21:57


Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57

Chemistry, 16.06.2020 21:57



Advanced Placement (AP), 16.06.2020 21:57

History, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57


English, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57

Mathematics, 16.06.2020 21:57



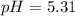
![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/0771/3232/33848.png)
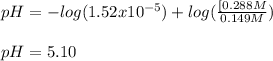
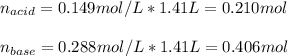
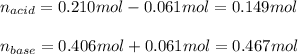
![[acid]=\frac{0.149mol}{1.41L} =0.106M](/tpl/images/0771/3232/f69f4.png)
![[base]=\frac{0.467mol}{1.41L} =0.331M](/tpl/images/0771/3232/b5538.png)