
Chemistry, 08.10.2020 01:01 Jasminehenry123
answer the questions below, using lt (for is less than), gt (for is greater than), eq (for equal to), or mi (for more information) in the blanks provided. 1. the wavelength of the photon required to promote an electron in the hydrogen atom from the n = 1 to the n = 3 level is the wavelength of the photon required to promote an electron in the hydrogen atom from the n =1 to the n = 2 level. 2. the energy of a photon with a wavelength of 463 nm is the energy of a photon whose wavelength is 722 nm. 3. in order to promote an electron to go to a higher energy level, light with a wavelength that is 400 nm is required.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
answer the questions below, using lt (for is less than), gt (for is greater than), eq (for equal to)...
Questions




















Mathematics, 07.09.2020 05:01

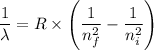
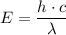
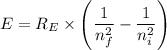
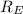 = -2.178 × 10⁻¹⁸ J
= -2.178 × 10⁻¹⁸ J 

