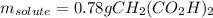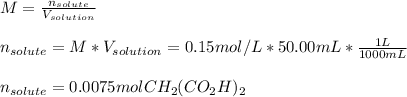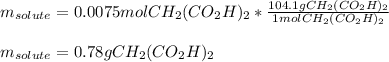Chemistry, 15.10.2020 02:01 marelinatalia2000
50.00 mL of a solution containing 0.15 M CH2 (CO2 H)2 and 0.020 M MnSO4
1. Calculate the mass in g of malonic acid required.
Watch your sig figs! (enter just the number)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
50.00 mL of a solution containing 0.15 M CH2 (CO2 H)2 and 0.020 M MnSO4
1. Calculate the mass in g...
Questions



Mathematics, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20



Geography, 08.12.2020 17:20


English, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20

Geography, 08.12.2020 17:20


English, 08.12.2020 17:20


Mathematics, 08.12.2020 17:20

Health, 08.12.2020 17:20

Mathematics, 08.12.2020 17:20


History, 08.12.2020 17:20