
Chemistry, 17.10.2020 15:01 Packergood
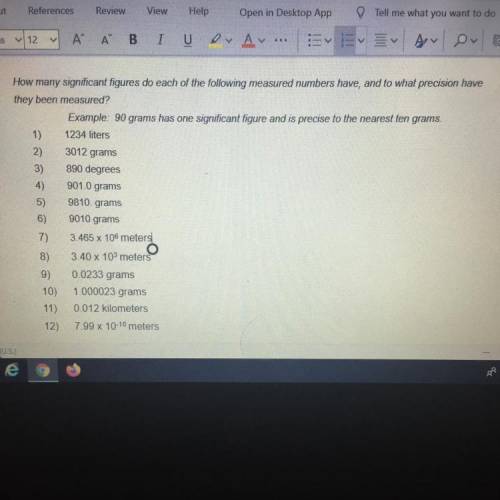
How many significant figures do each of the following measured numbers have, and to what precision have
they been measured?
Example: 90 grams has one significant figure and is precise to the nearest ten grams.
1) 1234 liters
2)
3)
4)
3012 grams
890 degrees
901.0 grams
9810. grams
9010 grams
5)
6)
7)
3.465 x 106 meters
8)
3.40 x 103 meters
9)
10)
0.0233 grams
1.000023 grams
0.012 kilometers
7.99 x 10-10 meters
11)
12)
U. S.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
How many significant figures do each of the following measured numbers have, and to what precision h...
Questions



Mathematics, 07.04.2021 01:00


Mathematics, 07.04.2021 01:00






Mathematics, 07.04.2021 01:00






Mathematics, 07.04.2021 01:00

Mathematics, 07.04.2021 01:00


History, 07.04.2021 01:00



