
Chemistry, 20.10.2020 03:01 hellokitty1647
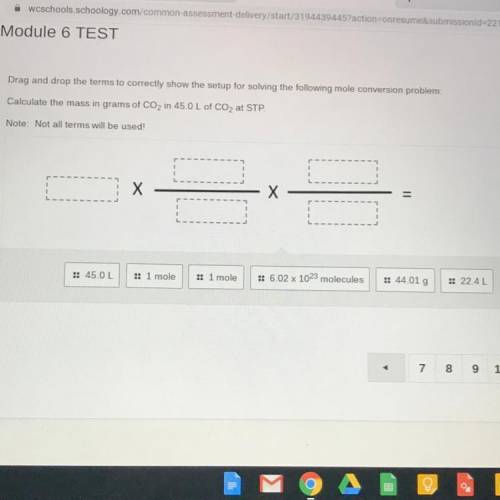
Drag and drop the terms to correctly show the setup for solving the following mole conversion problem:
Calculate the mass in grams of CO2 in 45.0 L of CO2 at STP.
Note: Not all terms will be used!
X
11
:: 45.0 L
:: 1 mole
: 1 mole
:: 6.02 x 1023 molecules
:: 44.01 g
: 22.4L

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

You know the right answer?
Drag and drop the terms to correctly show the setup for solving the following mole conversion proble...
Questions


Computers and Technology, 03.04.2020 17:30





Physics, 03.04.2020 17:31



History, 03.04.2020 17:31


History, 03.04.2020 17:31










