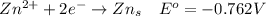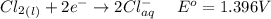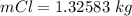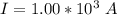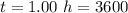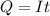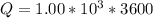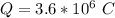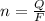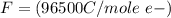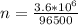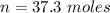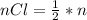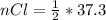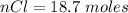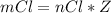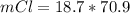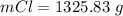Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reactions for each electrode. From which electrode will electrons flow from the battery into a circuit if the electrode potentials are not too different from E 8 values

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2


Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
Consider the rechargeable battery: Zn(s)0ZnCl (aq)7Cl2(aq)0Cl (l)0C(s) (a) Write reduction half-reac...
Questions


Mathematics, 20.09.2021 19:00

Social Studies, 20.09.2021 19:00

Mathematics, 20.09.2021 19:00



Mathematics, 20.09.2021 19:00

English, 20.09.2021 19:00




Mathematics, 20.09.2021 19:00



Mathematics, 20.09.2021 19:00


Mathematics, 20.09.2021 19:00

History, 20.09.2021 19:00


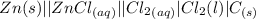
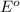 values
values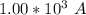 for 1.00 h , how many kg of
for 1.00 h , how many kg of 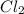 will be consumed
will be consumed