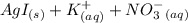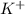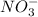When aqueous solutions of AgNO3 and KI are mixed, AgI precipitates. The balanced net ionic equation is . When aqueous solutions of AgNO3 and KI are mixed, AgI precipitates. The balanced net ionic equation is . AgNO3 (aq) + KI (aq) → AgI (s) + KNO3 (aq) Ag+ (aq) + I- (aq) → AgI (s) AgNO3 (aq) + KI (aq) → AgI (aq) + KNO3 (s) Ag+ (aq) + NO3 - (aq) → AgNO3 (aq) Ag+ (aq) + NO3 - (aq) → AgNO3 (s)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
When aqueous solutions of AgNO3 and KI are mixed, AgI precipitates. The balanced net ionic equation...
Questions

French, 16.12.2020 01:10


Mathematics, 16.12.2020 01:10



Chemistry, 16.12.2020 01:10


English, 16.12.2020 01:10



English, 16.12.2020 01:10




English, 16.12.2020 01:10

Spanish, 16.12.2020 01:10

Mathematics, 16.12.2020 01:10

Computers and Technology, 16.12.2020 01:10

Computers and Technology, 16.12.2020 01:10

Advanced Placement (AP), 16.12.2020 01:10

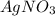 and
and 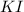 to form
to form 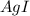 precipitates is: B.
precipitates is: B. 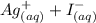 ----->
-----> 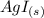
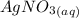 ----->
-----> 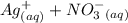
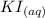 ----->
-----> 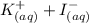
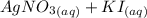 ----->
-----> 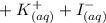 ----->
----->