Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

You know the right answer?
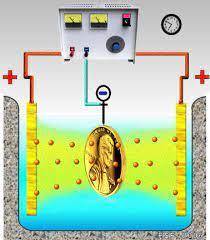
n a certain electroplating process gold is deposited by using a current of 14.0 A for 19 minutes. A...
Questions


Business, 03.02.2020 01:56

English, 03.02.2020 01:56

Health, 03.02.2020 01:56


Advanced Placement (AP), 03.02.2020 01:56

Social Studies, 03.02.2020 01:56


Spanish, 03.02.2020 01:56

English, 03.02.2020 01:56

History, 03.02.2020 01:56


Biology, 03.02.2020 01:56

Biology, 03.02.2020 01:56


Arts, 03.02.2020 01:56



Mathematics, 03.02.2020 01:56

Social Studies, 03.02.2020 01:56