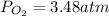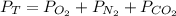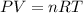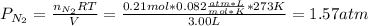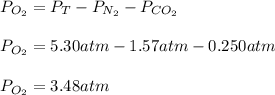Chemistry, 23.10.2020 15:40 kayleahrayne
Suppose that Daniel has a 3.00 L bottle that contains a mixture of O2 , N2 , and CO2 under a total pressure of 5.30 atm. He knows that the mixture contains 0.210 mol N2 and that the partial pressure of CO2 is 0.250 atm. If the temperature is 273 K, what is the partial pressure of O2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
Suppose that Daniel has a 3.00 L bottle that contains a mixture of O2 , N2 , and CO2 under a total p...
Questions


Social Studies, 09.10.2019 02:00




Chemistry, 09.10.2019 02:00


Mathematics, 09.10.2019 02:00


Mathematics, 09.10.2019 02:00

History, 09.10.2019 02:00

English, 09.10.2019 02:00


English, 09.10.2019 02:00




Biology, 09.10.2019 02:00

Biology, 09.10.2019 02:00

History, 09.10.2019 02:00