
Chemistry, 26.10.2020 17:00 datboyjulio21
If 102 grams of Sn(NO2)4 is reacted with 80.2 grams of Pt3N4, and yields 40.2 grams of Sn3N4. What is the limiting reagent in the reaction and what is the percent theoretical yield?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
If 102 grams of Sn(NO2)4 is reacted with 80.2 grams of Pt3N4, and yields 40.2 grams of Sn3N4. What i...
Questions

Mathematics, 25.02.2020 20:41






Mathematics, 25.02.2020 20:41



Mathematics, 25.02.2020 20:42



Mathematics, 25.02.2020 20:42




Geography, 25.02.2020 20:42


Business, 25.02.2020 20:42

Mathematics, 25.02.2020 20:42

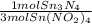 = 0.112 mol Sn₃N₄
= 0.112 mol Sn₃N₄

