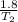Chemistry, 27.10.2020 21:30 mbonham481
A sample of Ar gas has a pressure of 1.20 atm at 125°C and a volume of 1.00 L. What would the temperature be (°C) with a pressure of 1.80 atm and constant volume?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2


Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
A sample of Ar gas has a pressure of 1.20 atm at 125°C and a volume of 1.00 L. What would the temper...
Questions

Mathematics, 20.11.2020 20:40

English, 20.11.2020 20:40

Arts, 20.11.2020 20:40



Mathematics, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

English, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

English, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40



Mathematics, 20.11.2020 20:40

Mathematics, 20.11.2020 20:40

Health, 20.11.2020 20:40

History, 20.11.2020 20:40


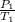 =
= 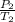
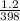 =
=