
Chemistry, 28.10.2020 17:00 kaylabustos10
Vitamin C was measured by an electrochemical method in a 50.0-mL sample of lemon juice. A detector signal of 2.02 mA was observed. A standard addition of 1.00 mL of 29.4 mM vitamin C increased the signal to 3.79 mA. Find the concentration (in mM units) of vitamin C in the juice.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Vitamin C was measured by an electrochemical method in a 50.0-mL sample of lemon juice. A detector s...
Questions


Computers and Technology, 31.01.2020 04:58


History, 31.01.2020 04:58


Chemistry, 31.01.2020 04:58

Chemistry, 31.01.2020 04:58

Mathematics, 31.01.2020 04:58








Computers and Technology, 31.01.2020 04:58

Chemistry, 31.01.2020 04:58

Spanish, 31.01.2020 04:58


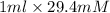
![\frac{[final concentration]}{[v_1/v_2]} \times[final concentration]+[concentration of sample])](/tpl/images/0847/5685/e9c49.png) or
or![\frac{[x]}{[v_1/v-2]} \times[ x]+[concentration of sample])](/tpl/images/0847/5685/7d567.png)


