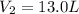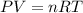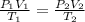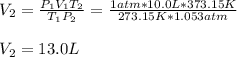Chemistry, 29.10.2020 03:50 mistiehaas
An ideal gas is confined to a 10.0-L balloon at STP. What is
the new volume of the balloon when it is placed under
800. mmHg at 100°C?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1


Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
An ideal gas is confined to a 10.0-L balloon at STP. What is
the new volume of the balloon when it...
Questions

Mathematics, 06.02.2021 21:20

Arts, 06.02.2021 21:20

Health, 06.02.2021 21:20



Mathematics, 06.02.2021 21:20

Computers and Technology, 06.02.2021 21:20



Mathematics, 06.02.2021 21:20

Mathematics, 06.02.2021 21:20

Mathematics, 06.02.2021 21:20

Mathematics, 06.02.2021 21:20


Mathematics, 06.02.2021 21:20

Health, 06.02.2021 21:20




Biology, 06.02.2021 21:20