
Chemistry, 06.11.2020 17:50 alexa006ox9k63
Determine the maximum amount of NaNO3 that was produced during the experiment. Explain how you determined this amount

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2


Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
Determine the maximum amount of NaNO3 that was produced during the experiment. Explain how you deter...
Questions


Mathematics, 04.11.2019 05:31

Chemistry, 04.11.2019 05:31


Mathematics, 04.11.2019 05:31


Chemistry, 04.11.2019 05:31

Mathematics, 04.11.2019 05:31

English, 04.11.2019 05:31

Mathematics, 04.11.2019 05:31

History, 04.11.2019 05:31

History, 04.11.2019 05:31




Advanced Placement (AP), 04.11.2019 05:31


History, 04.11.2019 05:31

Business, 04.11.2019 05:31

Biology, 04.11.2019 05:31

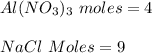
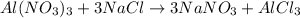
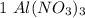 mole reacts in
mole reacts in 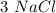 moles to give
moles to give 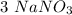 moles and
moles and 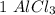 mole.
mole. 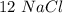 moles,
moles, 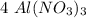 moles react totally. And though we got
moles react totally. And though we got 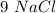 moles.
moles. 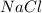 is the only reagent.
is the only reagent. 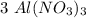 moles to give
moles to give 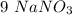 moles and
moles and 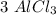 moles.
moles. 


