Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
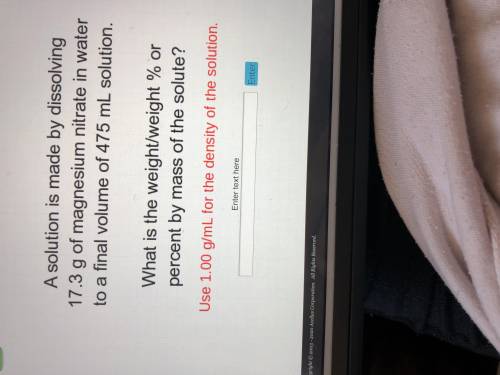
A solution is made by dissolving 17.3 g of magnesium nitrate in water to a final volume of 475 mL so...
Questions

Mathematics, 04.08.2019 07:00


Mathematics, 04.08.2019 07:00



Mathematics, 04.08.2019 07:00




Mathematics, 04.08.2019 07:00


Social Studies, 04.08.2019 07:00

Chemistry, 04.08.2019 07:00

Biology, 04.08.2019 07:00





English, 04.08.2019 07:00

Spanish, 04.08.2019 07:00