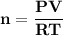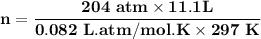Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
A steel cylinder for scuba diving contains 11.1 L of compressed air. The pressure inside the cylinde...
Questions

Mathematics, 27.09.2020 19:01


Biology, 27.09.2020 19:01

English, 27.09.2020 19:01



History, 27.09.2020 19:01

Mathematics, 27.09.2020 19:01




Physics, 27.09.2020 19:01


Mathematics, 27.09.2020 19:01

Mathematics, 27.09.2020 19:01

Spanish, 27.09.2020 19:01


Geography, 27.09.2020 19:01

History, 27.09.2020 19:01

Mathematics, 27.09.2020 19:01