Select the correct answer.
What inference can be drawn from the graph?
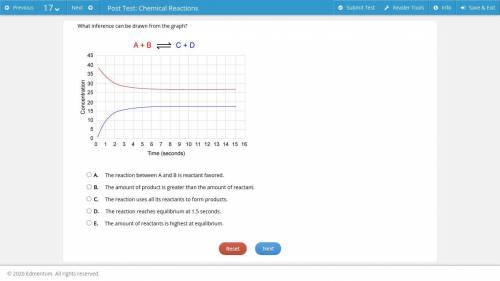
a graph of react...

Chemistry, 23.11.2020 03:20 asiamuhammad6
Select the correct answer.
What inference can be drawn from the graph?
a graph of reactant and product concentration vs. time; a red curve shows the reactant decreasing and a blue curve shows the product concentration increasing over time; the two curves do not intersect; the chemical equation shows A + B reacting to produce C + D
A.
The reaction between A and B is reactant favored.
B.
The amount of product is greater than the amount of reactant.
C.
The reaction uses all its reactants to form products.
D.
The reaction reaches equilibrium at 1.5 seconds.
E.
The amount of reactants is highest at equilibrium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Questions

Health, 18.12.2019 03:31


Mathematics, 18.12.2019 03:31

English, 18.12.2019 03:31

Advanced Placement (AP), 18.12.2019 03:31



Mathematics, 18.12.2019 03:31



Biology, 18.12.2019 03:31

History, 18.12.2019 03:31

Health, 18.12.2019 03:31



Mathematics, 18.12.2019 03:31

Social Studies, 18.12.2019 03:31





