
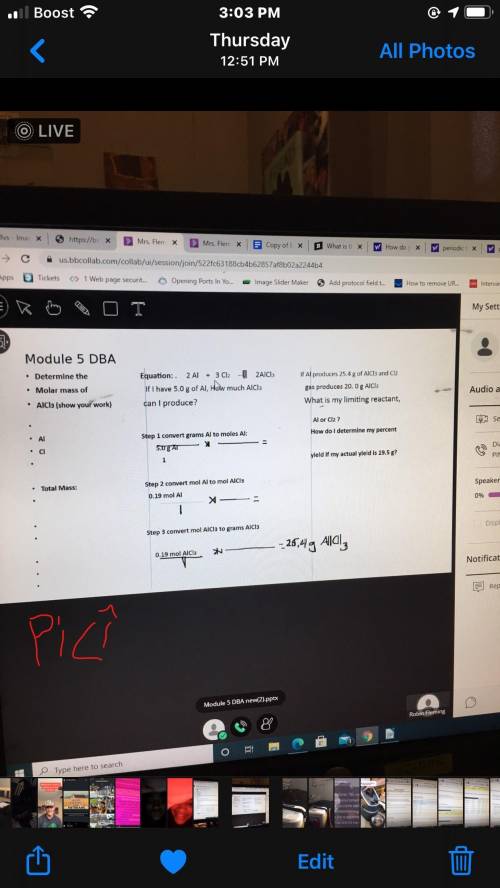
2Al+3Cl2---> 2AlCl3 (i cant remember if this is balanced equation or not if not can you do that too? thank you i appreciate it.)
If I have 5.0 g Aluminum how much AlCl can I produce?
Step 1 convert grams Al to moles Al: (the dashes resemble a fraction)
5.0 g Al
- times - =
Step 2: convert mol al to mol AlCl3
0.19 mol Al
- times - =
1
step 3: convert MOL AlCl3 to GRAMS AlCl3
0.19 mol AlCl3
- times - = 25.4 g AlCl3
1
THIS FIRST # PART PROBLEM IS STOICHEMITRY for chemistry if you know please help me out I have been struggling on this for a week now..thank you so much.
This next part is LIMITING REACTANTS:
If Al produces 25.4 g of AlCl3 and Cl2 gas produces 20.0 g AlCl3 what is my limiting reactant, Al or C2??
How do i determine my percent yeild if my actual yeild is 19.5g???
i put a link to the picture!!

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1



You know the right answer?
2Al+3Cl2---> 2AlCl3 (i cant remember if this is balanced equation or not if not can you do that t...
Questions

Computers and Technology, 04.12.2019 02:31

Business, 04.12.2019 02:31

Biology, 04.12.2019 02:31

Mathematics, 04.12.2019 02:31

English, 04.12.2019 02:31


Mathematics, 04.12.2019 02:31

Mathematics, 04.12.2019 02:31



Biology, 04.12.2019 02:31







Mathematics, 04.12.2019 02:31





