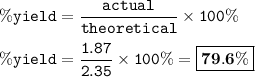Chemistry, 29.11.2020 08:50 zitterkoph
Can someone please help me with this?-- 18 pts!
2C10H11NO4 + 2H2O → C16H10N2O2 + 2C2H4O2 + 4H2O
In this reaction, 1.87 g of indigo (C16H10N2O2) are produced.
If the theoretical yield is 2.35 g of C16H10N2O2, what is the percent yield of indigo?
Round to the nearest decimal.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1
You know the right answer?
Can someone please help me with this?-- 18 pts!
2C10H11NO4 + 2H2O → C16H10N2O2 + 2C2H4O2 + 4H2O
Questions


Computers and Technology, 21.05.2020 23:19









English, 21.05.2020 23:19

English, 21.05.2020 23:19