Chemistry, 03.12.2020 07:00 alexreddin3127
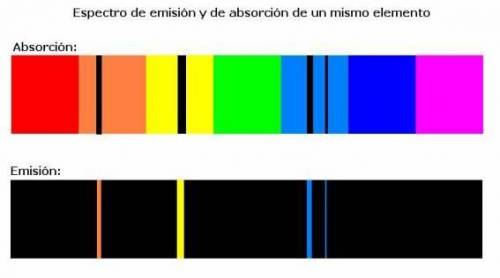
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not quantizied
a. lines would be shifted into the ultraviole region
b. there would be fewer lines
c. there will be more lines
d. the spectrum would be constnuous

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not q...
Questions







Social Studies, 16.03.2020 20:55



Computers and Technology, 16.03.2020 20:55


History, 16.03.2020 20:55


Mathematics, 16.03.2020 20:55

History, 16.03.2020 20:55

Mathematics, 16.03.2020 20:55

English, 16.03.2020 20:55



Biology, 16.03.2020 20:55