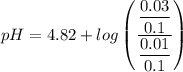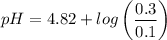Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2


Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Calculate the pH of a buffer when 0.010 moles of NaOH is added to 100. mL solution that is 0.20 M so...
Questions




















![pH = pKa + log \dfrac{[salt]}{[Acid]}](/tpl/images/0960/5001/27039.png)
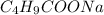 = 0.20 M
= 0.20 M 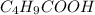 = 0.20 M
= 0.20 M