
Chemistry, 12.12.2020 17:10 jakhunter354
Indicate whether aqueous solutions of each of the following solutes will contain only ions, only molecules, or mostly molecules and a few ions:
a) NH4Cl, a strong electrolyte.
b) Ethanol, CH3-CH2-OH, a nonelectrolyte.
c) HCN, hydrocyanic acid, a weak electrolyte.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Indicate whether aqueous solutions of each of the following solutes will contain only ions, only mol...
Questions

Mathematics, 21.05.2021 03:00

Mathematics, 21.05.2021 03:00


Mathematics, 21.05.2021 03:00



SAT, 21.05.2021 03:00

History, 21.05.2021 03:00

Mathematics, 21.05.2021 03:00

Biology, 21.05.2021 03:00

Social Studies, 21.05.2021 03:00



Mathematics, 21.05.2021 03:00


Mathematics, 21.05.2021 03:00

English, 21.05.2021 03:00

Mathematics, 21.05.2021 03:00

Mathematics, 21.05.2021 03:00

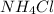 has a strong electrolyte.
has a strong electrolyte.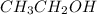 , it has a nonelectrolyte.
, it has a nonelectrolyte.


