

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

You know the right answer?
When hydrogen reacts with calcium metal, what are the oxidation numbers of the calcium and hydrogen...
Questions

Computers and Technology, 14.01.2020 19:31

History, 14.01.2020 19:31


History, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31




Spanish, 14.01.2020 19:31



Health, 14.01.2020 19:31





Mathematics, 14.01.2020 19:31



Mathematics, 14.01.2020 19:31


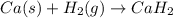
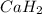 , it is -2.
, it is -2.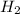 is zero and oxidation number of hydrogen in
is zero and oxidation number of hydrogen in 

