Chemistry, 17.12.2020 09:50 xochitlbmartine
Use the reaction given below to solve the problem that follows: Calculate the mass in grams of aluminum oxide produced by the reaction of 15.0 g of aluminum metal.
[ ]grams Al2O3
4 Al + 3 O2 --> 2 Al2O3
**Your answer should be written as XX. X

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
You know the right answer?
Use the reaction given below to solve the problem that follows: Calculate the mass in grams of alumi...
Questions

Biology, 08.12.2020 01:00

History, 08.12.2020 01:00




Mathematics, 08.12.2020 01:00



English, 08.12.2020 01:00

Computers and Technology, 08.12.2020 01:00








Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

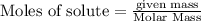
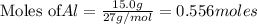
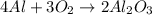
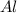 produce == 2 moles of
produce == 2 moles of 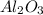
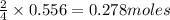 of
of