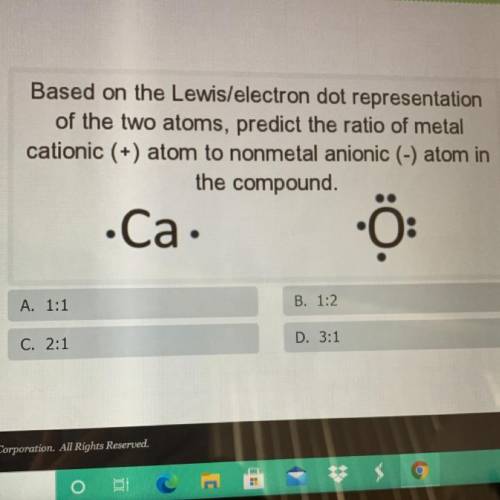
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
c...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Questions

English, 05.05.2020 00:27

Mathematics, 05.05.2020 00:27

Chemistry, 05.05.2020 00:27




Chemistry, 05.05.2020 00:27


English, 05.05.2020 00:27






Mathematics, 05.05.2020 00:27

Mathematics, 05.05.2020 00:27


Biology, 05.05.2020 00:27


Mathematics, 05.05.2020 00:27